Nanomaterials can be defined as materials possessing at least one external dimension that measures 100 nanometres or less or with internal structures measuring 100 nm or less.
They may be in the form of particles, tubes, rods or fibres. Nanomaterials can occur naturally, be produced purposefully through engineering or be created as the by-products of combustion reactions to perform a specialized function.
Characterization of Nanomaterials
Physical Characterization Includes
- Shape, size, specific surface area, and ratio of width and height.
- Whether they stick together.
- Number size distribution and structure, including crystal structure and any crystal defects.
- How smooth or bumpy their surface is and how well they dissolve.
Chemical Characterization Includes
- Molecular structure, composition (purity, and impurity or additive) and surface chemistry.
- Whether it is held in a solid, liquid or gas, and attraction to water molecules or oils and fats.
Properties of Nanomaterials
| Properties | Details |
|---|---|
| Mechanical | Nanomaterials show different mechanical properties due to the increased number of surface atoms and interfaces. This includes increased strength, toughness, hardness, ductility and decreased elasticity. |
| Thermal | Increasing the number of grain boundaries enhances phonon scattering at the disordered boundaries and results in lower thermal conductivity. Thus, nanomaterials have lower thermal conductivity compared to conventional materials. |
| Electrical | Nanomaterials have lower electrical conductivities than bulk materials. They have a high density of grain boundaries, which makes electric-phonon and phonon-phonon scattering effective and reduces conductivity. |
| Magnetic | Materials with nanostructures have higher saturation magnetization and magnetic coercivity values. Nanoparticles become magnetic in the presence of an external magnet, but revert to a nonmagnetic state when the external magnet is removed. |
| Melting Point | Melting-point depression phenomenon is very prominent in nanomaterials. They melt at temperatures lower than bulk materials. |
| Optical | Nanomaterials exhibit distinctive optical characteristics such as absorption, transmission, reflection, greater scattering, and light emission. The shape and size of nanoparticles can be altered to change their optical properties. |
Types of Nanomaterials
- Inorganic-based nanomaterials:
Include different metal and metal oxide nanomaterials.- Examples of metal-based inorganic nanomaterials:
Silver, gold, cadmium, copper, iron, zinc etc. - Examples of metal oxide-based inorganic nanomaterials:
Zinc oxide, copper oxide, magnesium aluminum oxide, titanium dioxide, iron oxide, etc.
- Examples of metal-based inorganic nanomaterials:
- Carbon-based nanomaterials:
It includes nanomaterials made up of non-metals and thus include graphene, fullerene, single-walled carbon nanotube, multiwalled carbon nanotube, carbon fibre, an activated carbon, and carbon black. - Organic-based nanomaterials:
Formed from organic materials excluding carbon materials, for instance, dendrimers, liposomes, etc. - Composite nanomaterials:
They are any combination of metal-based, metal oxide-based, carbon-based, and/or organic-based nanomaterials. These nanomaterials have complicated structures like a metal-organic framework.
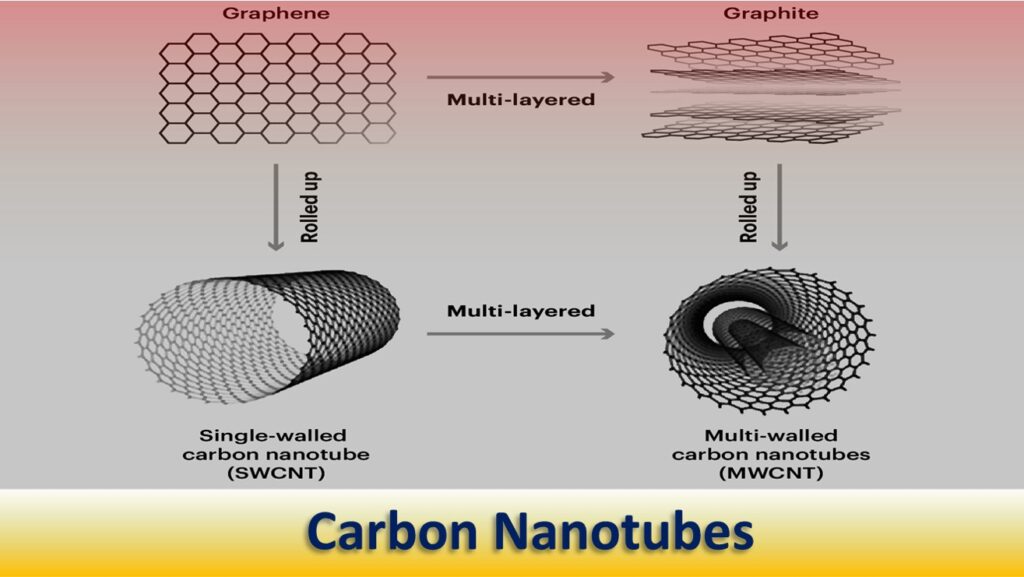
Carbon Nanotube (CNT)
Carbon Nano-tubes (also known as Bucky-tube) are an allotrope of carbon. These are hollow, tubular and caged molecules, having a diameter measuring on the nanoscale.
They are made up of continuous unbroken hexagonal mesh with carbon molecules at the apexes of the hexagons.
The bonding in carbon nanotubes is sp², with each carbon atom joined to three neighbors, as in graphite. The tubes can therefore be considered as rolled-up graphene sheets (graphene is an individual graphite layer).
CNTs are of two types: single-wall carbon Nano-tubes (SWCN) and multiple-wall carbon Nano-tubes (MWCN).
These cylindrical carbon molecules have unusual properties, which are valuable for nanotechnology, electronics, optics and materials science and technology.
Properties of CNT
- Mechanical Properties of CNT:
CNT are the strongest and stiffest materials on earth in terms of tensile strength (ability to withstand a stretching force without breaking). They are hundred times stronger, yet six times lighter than steel. They are one of the hardest substances known; a standard single-walled carbon nanotube can withstand a pressure up to 24GPa without deformation. - Electrical Conductivity of CNT:
CNT can carry 1000 times more electric current than equivalent copper & silver wire, so is regarded as an ideal component for electric circuits. - Thermal Conductivity of CNT:
CNT are very good thermal conductors and the temperature stability of CNT is up to 2800℃ in vacuum & 780℃ in air.
Application of CNT
- Because of their extraordinary thermal and electrical conductivity and mechanical properties, carbon nano-tubes find applications in a wide range of materials.
- For instance, computer’s processor and memory, electronic equipment, transistors, ultra-capacitors, solar cells, baseball bats, golf clubs, or car parts, combat jackets, cancer treatment and detection, etc.
Graphene
Graphene is a two dimensional allotrope of carbon. It is one atom thick, planar and hexagonal arrangements of carbon atoms.
The bonding of graphene is sp², with each carbon atom joined to three neighbours, as in graphite.
It can be wrapped up into ‘zero-dimensional’ fullerenes, rolled into ‘one-dimensional’ nanotubes or stacked into ‘three-dimensional’ graphite. The word ‘Graphene’ came from graphite.
In 2004, teams including Andre Geim and Konstantin Novoselov separated graphene from graphite and demonstrated that single layers of graphite could be isolated, resulting in the award of the Nobel Prize for Physics in 2010.
Properties of Graphene
It is a good thermal and electric conductor and can be used to develop semiconductor circuits and computer parts. Experiments have shown it to be incredibly strong and hard.
Applications of Graphene
- Its unusual properties make it ideal for applications in various fields from composite materials to electronics.
- Graphene is a transparent conductor which can be used in touch-screen & light panel displays in smart phones & tablets. It can also be used in the making of solar cells.
- Graphene transistors are faster than those made out of silicon.
- Graphene components could pack a chip more tightly and can help make efficient and fast computers.
- Plastics could be made into electronic conductors, if only 1% of graphene were mixed into them, which will also increase the heat resistance and will make it mechanically robust.
- Graphene also helps in making new super strong materials which are thin, elastic & light weight; which can be used to make components of airplanes, cars and satellites.
- It can also be used in making many chemical and biological sensors to be used in healthcare, environmental and industrial processes.
Fullerenes
Fullerenes are a carbon allotrope. They are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube.
Spherical fullerenes are called buckyballs whereas cylindrical fullerenes are called buckytubes or nanotubes.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they also contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
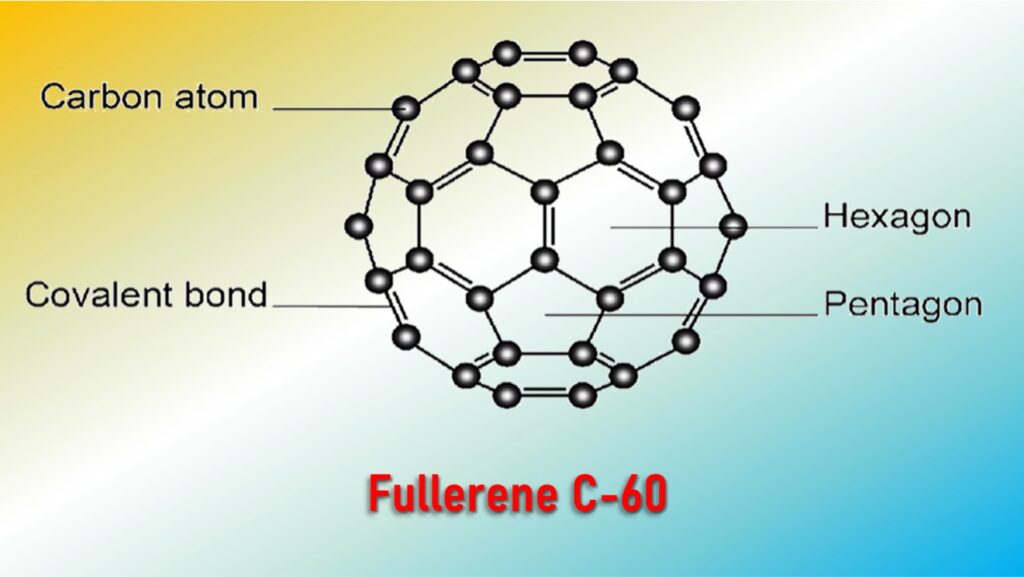
Buckminster Fullerenes
The C-60 fullerene is the most stable and was the first to be identified. It contains 60 carbon atoms which are arranged in the shape of a football.
It contains 20 six-member hexagonal rings and 12 five-member pentagonal rings.
C-60 fullerene look like geodesic domes designed by the United States architect Buckminster Fuller, therefore, they are called Buckminster Fullerenes.
Properties of C-60
- Buckminster Fullerenes (C-60) is a dark solid at room temperature and its hardness lies between diamond and graphite. It is neither very hard nor very soft.
Application of C-60
- It is a strong antioxidant; enough to kill resistant bacteria and cancer cells in the human body.
- C-60 molecules can engage and transport atoms and molecules (e.g. drugs, radioactive labels) through the human body.
- C-60 could also inhibit the HIV virus. The C60 molecule could block the active site in a key enzyme in the human immunodeficiency virus known as HIV-1 protease; this could inhibit reproduction of the HIV virus in immune cells.
- The C-60 molecule can also bind large numbers of hydrogen atoms without disrupting the structure. This property suggests that C60 may be a better storage medium for hydrogen than metal hydrides and hence a key factor in the development of a new class battery or even non-polluting automobiles.
- It shows the properties of superconductivity and thermal resistance which makes it perfect to be used in electronic engineering.
Carbon Fibres
Carbon fibres can be defined as fibers with a carbon content of 90% or above.
They are produced by thermal conversion of organic fibers with a lower carbon content such as polyacrylonitrile (PAN) containing several thousand filaments with diameter between 5 and 10 μm.
Carbon fibers have high tensile strength, high stiffness, low density, and a high chemical resistance.
The main application areas of carbon fiber-reinforced polymers are aerospace, defense, automotive, wind turbines, sport and leisure, and civil engineering.
Also Read
Applications of Nanomaterials in Medical and Healthcare industries
What is Nanoscale objects and its Behavior
Basic Concepts on Nanotechnology, Nanoscale, Nanomaterials, Nanoscience and Nanoengineering









2 thoughts on “Types of Nanomaterials and its properties in details”