What is Nuclear Fusion reaction?
Nuclear fusion is a process by which nuclear reactions between light elements form heavier elements (up to iron).
In cases where the interacting nuclei belong to elements with low atomic numbers (e.g., hydrogen [atomic number 1] or its isotopes deuterium and tritium), substantial amounts of energy are released.
Fusion is the process that powers active stars. The energy released from nuclear fusion reactions accounted for the longevity of the Sun and other stars as a source of heat and light.
The prime energy producer in the Sun is the fusion of hydrogen to form helium. It takes four hydrogen atoms to fuse into each helium atom. During the process some of the mass is converted into energy.
Fusion Reaction
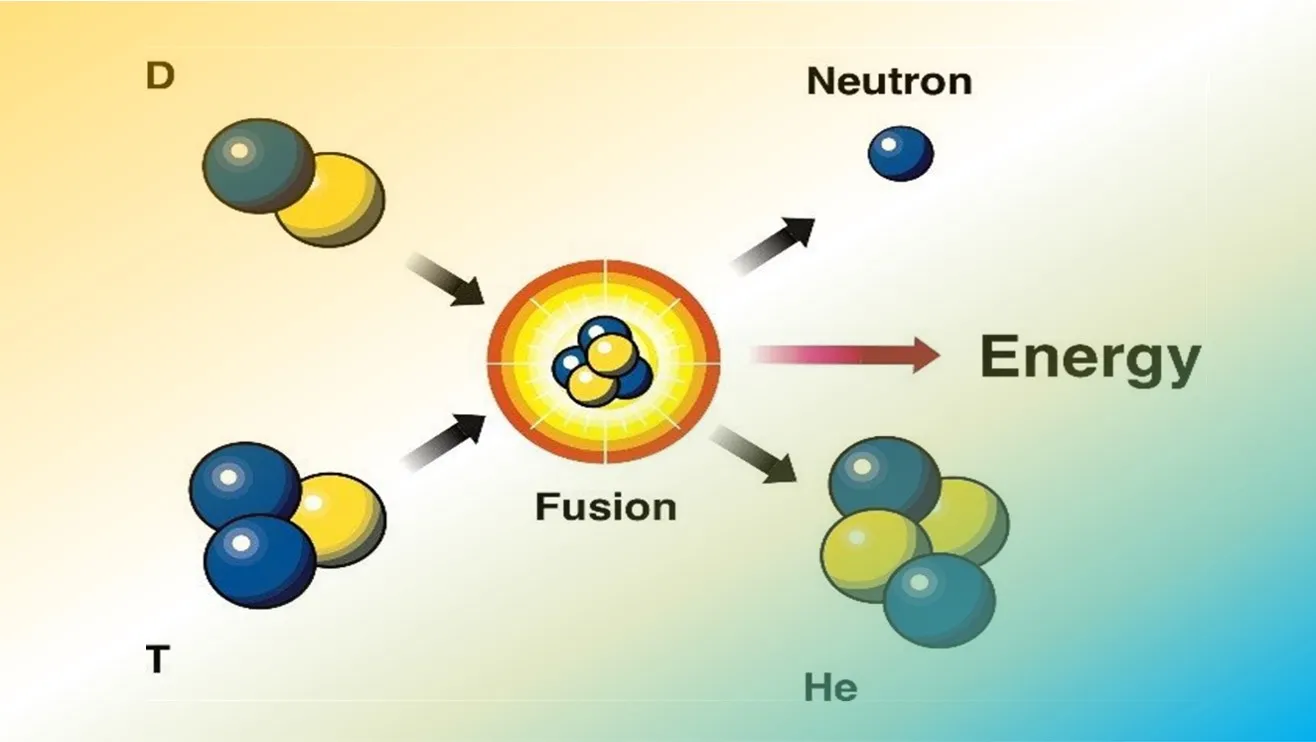
An important fusion reaction for practical energy generation is that between deuterium and tritium (the D-T fusion reaction). It produces helium (He) and a neutron (n) and is written:
D + T → He + n
Fusion reactions between light elements release energy because of the mass difference between the Z protons and N neutrons considered separately and the nucleons bound together (Z + N) in a nucleus of mass M.
M is less than (Z + N). The mass difference is converted into energy under the equation (E=MC2).
Two Basic Types of Fusion Reactions
- Those that preserve the number of protons and neutrons: Most important for practical fusion energy production.
- Those that involve a conversion between protons and neutrons: Crucial to the initiation of star burning.
Principle Nuclear Fusion reactions
Fusion reactions are inhibited by the electrical repulsive force, called the Coulomb force, that acts between two positively charged nuclei.
For fusion to occur, the two nuclei must approach each other at high speed in order to overcome their electrical repulsion and attain a sufficiently small separation (less than one-trillionth of a centimetre) so that the short-range strong force dominates.
For the production of useful amounts of energy, a large number of nuclei must undergo fusion; that is to say, a gas of fusing nuclei must be produced.
In a gas at extremely high temperatures, the average nucleus contains sufficient kinetic energy to undergo fusion.
Such a medium can be produced by heating an ordinary gas beyond the temperature at which electrons are knocked out of their atoms. The result is an ionized gas consisting of free negative electrons and positive nuclei.
This ionized gas is in a plasma state, the fourth state of matter. Most of the matter in the universe is in the plasma state.