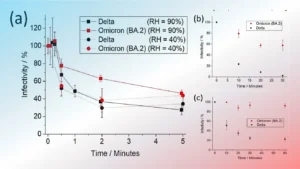
What is Biosafety?
Biosafety is a broad term and its means 2 things:
- In general practice: Biosafety is the safe working practices associated with handling of biological materials, particularly infectious agents.
- In the context of biological diversity: Biosafety refers to protecting the native biological diversity from aggressive invasive species including living-modified organisms. Its main objective is to keep a check on harmful biological agents, toxins, chemicals, and radiation.
Biosafety Aspects of Genetically Modified Crops in India
The Government of India has very strict guidelines to test and evaluate the agronomic value of the GM crops so as to protect the interests of the farmers. These guidelines address all concerns with regard to the safety of GM seeds.
The regulatory system for GM crops as operative in the Department of Biotechnology, Ministry of Science and Technology and Ministry of Environment and Forests has guidelines to consider the GM crops on a case-by-case basis towards testing.
Genetic Engineering Appraisal Committee (GEAC)
Genetic Engineering Appraisal Committee (GEAC) has its primary responsibility in the field of ensuring biosafety. It works under the Ministry of Environment, Forests and Climate Change (MoEFCC).
The Committee functions as a Statutory Body for approval of activities involving largescale use of hazardous living microorganisms and recombinants in research and industrial production from the environmental angle as per the provisions of rules 1989.
The Committee is responsible for approval of proposals relating to release of genetically engineered organisms and products into the environment including experimental field trials as per the provisions of Rules, 1989.
The Committee is also responsible for approval of proposals involving the use of living modified organisms (LMOs) falling in the risk category III and above in the manufacture/import of recombinant pharmaceutical products or where the end product of the recombinant pharmaceutical products per se are LMOs.
Main functions of GEAC
- To permit the use of GMOs and products thereof for commercial applications.
- To adopt producers for restriction or prohibition, production, sale, import & use of GMOs both for research and applications under the Environment Protection Act (EPA), 1986.
- To approve for conduct of large scale field trials, evaluation of large scale field trial data and final approval for release of transgenic crops into the environment.
- To authorise large scale production and release of GMOs and products thereof into the environment.
- To authorise agencies or persons to have powers to take punitive actions under the EPA.
Rules 1989
For facilitating and regulating the research work on GMOs & products thereof at laboratory scale and also in commercial applications, the Government of India notified “ Rules for the Manufacture/Use/Import/Export and Storage of Hazardous Microorganisms, Genetically Engineered Organisms or Cells ” in 1989 under the provisions of Environment (Protection) Act, 1986 through the Ministry of Environment & Forests (MoEF). These rules are commonly referred to as ‘ Rules 1989’.
These rules are implemented by MoEFCC, the Department of Biotechnology (DBT), Ministry of Science and Technology and the State Governments through the six competent authorities notified under the Rules which are as follows:
- Recombinant DNA Advisory Committee (RDAC)
- Institutional Biosafety Committee (IBSC)
- Review Committee on Genetic Manipulation (RCGM)
- Genetic Engineering Appraisal Committee (GEAC)
- State Biotechnology Coordination Committee (SBCC)
- District Level Committee (DLC).
Scope of Rules, 1989
These rules are very broad in scope essentially covering the entire spectrum of activities involving GMOs and products thereof.
They also apply to any substances, cells, tissues, products, and foodstuffs, etc.
New gene technologies apart from genetic engineering have also been included.
Mandate of Rules, 1989
In accordance with Rules, RCGM shall function from the Department of Biotechnology to monitor the safety-related aspect in respect of ongoing research projects or activities involving hazardous microorganisms, GE organisms and cells and products thereof.
RCGM shall bring out manuals of guidelines specifying procedure for regulatory process with respect to activities involving GE organisms in research use as well as industrial & environmental applications with a view to ensure human health and environmental safety.
All ongoing research projects involving hazardous microorganisms, GE organisms or cells and products thereof shall be reviewed to ensure that adequate precautions and containment conditions are being met.
RCGM shall lay down procedures restricting or prohibiting the production, sale, importation and use of such hazardous microorganisms, GE organisms or cells.
Cartagena Protocol on Biosafety
Cartagena Protocol on Biosafety is a legally binding, international agreement, supplemental to the Convention on Biological Diversity.
The Protocol seeks to protect biological diversity by managing the movements of Live Modified Organisms (LMOs) between countries.
The objective of the Cartagena Protocol is to contribute to ensuring an adequate level of protection in the field of the safe transfer, handling and use of LMOs resulting from modern biotechnology that may have adverse effects on the conservation and sustainable use of biological diversity, taking also into account risks to human health, and specifically focusing on transboundary movements.
It was adopted on 29 January 2000 as a supplementary agreement to the CBD and entered into force on 11 September 2003.
India is a signatory to the Cartagena Protocol on Biosafety and ratified it on January 23, 2003.
What does the Biosafety Protocol do?
It establishes an Advance Informed Agreement (AIA) procedure for ensuring that countries are provided with the information necessary to make informed decisions before agreeing to the import of such organisms into their territory.
It contains the precautionary approach of the Rio Declaration on Environment and Development. The Protocol provides for practical requirements that are deemed to contribute to the safe movement of LMOs.
The Protocol also establishes a Biosafety Clearing-House (BCH) to facilitate the exchange of information on living modified organisms and to assist countries in the implementation of the Protocol.
Also Read:
What is Reproductive Cloning and How does it works?